Can the Ammonia Cycle be Decarbonized?
The Clean Ammonia Ecosystem, Part 2
In part 1 of this trilogy, I examined whether and how ammonia could become the dominant energy carrier for a future carbon-free energy economy. In part 3, I will dig deeper into the clean ammonia ecosystem to look at a couple of promising applications, showing how ammonia closes its own production and consumption loops, generating clean energy along the way. If you like, follow along in the system map I used to help write this trio of articles.
Here in part 2, we need to understand whether and how ammonia used for energy can be produced cleanly—without greenhouse-gas emissions or other forms of pollution—at scales never before imagined.
In part 1, I mentioned that ammonia demand for electricity generation through gas turbines alone would represent a 2000-fold increase over current ammonia production for industrial uses. That is the scale of the challenge facing ammonia as an energy carrier. Can it be done, technically speaking? If it can, then can it be done without placing a significant burden on nature’s capacity to support the ammonia ecosystem? Or is it possible, perhaps, that in ‘ammonizing’ the energy economy, the burden on natural capacity actually would be lessened?
Ammonia’s carbon footprint
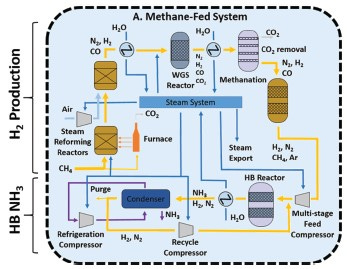
Almost all of the roughly 175 million tonnes of ammonia produced annually for industrial purposes today[1] is made from hydrocarbons using the Haber-Bosch process, first commercialized in 1910 by Carl Bosch at BASF using a technique developed a year earlier by Fritz Haber. A conventional Haber-Bosch process consists of three stages: (1) extraction of nitrogen from the air, (2) extraction of hydrogen from a hydrogen carrier, and (3) reaction of the two to form ammonia at high temperature and pressure over a magnetite catalyst.
N2 + 3H2 ⇌ 2NH3 + heat
The reaction is exothermic, producing about 5400 kilojoules of energy per kilogram of ammonia, which equates to about 1.5 kilowatt-hours. This useful heat can be fed back into the ammonia process or used for other purposes.

The air-separation process, which produces nitrogen, is typically performed by cryogenic partial distillation of nitrogen and oxygen from air, the oxygen going to other uses. The process is quite energy-intensive, requiring between 0.37 kWh and 0.55 kWh per kilogram of N2 produced.[2] Adsorption techniques for nitrogen extraction from air are more energy-efficient but they do not produce the required purity. Impurities in the nitrogen feed can contaminate the Haber-Bosch catalyst.
Hydrogen carriers conventionally used in the Haber-Bosch process are coal or natural gas.
Coal is combusted in a gasifier from whose emissions stream particulates and compounds of sulfur are removed. The output, which contains a mixture of hydrogen and carbon monoxide, is known as synthesis gas, or syngas. This is fed into a water gas-shift reactor, which extracts additional hydrogen in the presence of steam and a catalyst. The by-product is carbon dioxide, which is either emitted or captured.
Reaction 1: C24H12 + 12O2 ⇌ 24CO + 6H2
Reaction 2: CO + H2O ⇌ CO2 + H2.
One molecule of hard coal yields 30 molecules of hydrogen by this process, along with 24 molecules of CO2. It is both productive and polluting.
More commonly these days, hydrogen is produced by steam reformation of natural gas, which is a somewhat cleaner process, but with a lower yield. Methane reacts with high-temperature steam in the presence of a catalyst to produce carbon monoxide and hydrogen.
CH4 + H2O ⇌ CO + 3H2
The CO is then fed into the same water gas-shift reaction as in coal gasification.[3] The process yields four molecules of hydrogen for every molecule of methane, or about 13% of the yield of coal gasification. It emits of one molecule of CO2, which equals 30% of the emissions of coal gasification.
The streams of pure N2 and H2 from these processes are then fed into a Haber-Bosch reactor, which combines them catalytically at temperatures above 400°C and pressures over 200 bar (3000 psi). The Haber-Bosch process is energy-intensive, requiring a net input of about 8 kWh per kilogram of ammonia produced.[4] Electricity for the Haber-Bosch reactor usually is generated on-site from the steam loop, whose heat is provided by the hydrogen separation process. Including the upstream processes, the overall energy intensity of ammonia production is about 8.3 kWh per kilogram. Ammonia’s lower heating value (available net energy) is 5.2 kWh per kilogram, so the overall energy efficiency of ammonia via the Haber-Bosch process is just over 60 percent.
The schematic below summarizes Haber-Bosch using natural gas reformation.
The process’s energy cost, efficiency and emissions act as reference points against which alternate pathways for clean ammonia can be compared.
Cleaning up Haber-Bosch
Existing Haber-Bosch plants can be substantially improved with the addition of carbon capture and storage (CCS), an area of active development in recent years. In its 2018 budget, the US Congress approved a credit of $10 to $50 per tonne of CO2 captured and stored, depending on the method of sequestration or reuse. Few ammonia plants are retrofitted with CCS as yet, although CF Industries announced in 2022 a project to capture two million tons per year of CO2 at its Donaldson Nitrogen Complex in Louisiana.
Where coal gasification emits about 3 tonnes of CO2 per tonne of ammonia produced, and steam reformation of natural gas emits about 1.6 tonnes, CCS will cut emissions to about 0.2 tonnes of CO2 per tonne of ammonia.[5] There’s an energy cost to CCS, although it is modest relative to the Haber-Bosch process itself.
Since air separation to produce nitrogen relies on electricity, its carbon footprint is largely a function of the fuel mix of delivered power. Decarbonizing air separation is, in principle, as simple as powering it from renewable or other carbon-free sources. The key to cleaning up ammonia production through the Haber-Bosch process is in decarbonizing the production of hydrogen.[6] Among the many emerging technologies, both for ammonia production and other applications, several show early promise, described below in descending order of technological readiness. All can be powered by renewable or other carbon-free energy and, as such, would be eligible for government incentives, such as under the Inflation Reduction Act in the US. Others are just emerging from the lab.
Water electrolysis (current technologies)
The most widespread and well-developed fossil-free technology for extracting hydrogen is water electrolysis, the splitting of water with an electric current. For this, clean, filtered freshwater is needed: impurities would gum up the electrodes. Industrial-scale hydrogen production from water requires a large, reliable water supply. Hypothetically, if ammonia were to replace natural gas for power generation then about 300 billion tonnes of it would be needed annually. This requires about 53 billion tonnes of hydrogen, by atomic weight, or 159 billion tonnes of water, equal to 159 km3. That’s about the volume of Lake Tahoe. It’s a drop in the bucket of all available surface freshwater (0.00015%, to be precise) but still significant.
Where freshwater is neither plentiful nor reliable, water electrolysis plants would need to be located near the coast, or offshore, to rely on desalinated seawater. Although desalination itself carries an extra energy cost, it is small compared to that of water electrolysis.[7]
Two main technologies are currently used.
In proton-exchange membrane (PEM) water electrolysis, water is split in a cell equipped with a solid polymer electrolyte responsible for the conduction of protons, the separation of product gases, and electrical insulation of the electrodes. Its electrical efficiency is around 80 percent.
In alkaline water electrolysis, an electrolyzer having two electrodes operates in a liquid alkaline electrolyte, typically potassium hydroxide (KOH) or sodium hydroxide (NaOH). A diaphragm separates the electrodes, thereby keeping the product gases separate, and allowing transport of hydroxide ions (OH−) from one electrode to the other. Its advantages over PEM electrolysis include cheaper catalysts, higher durability and higher gas purity.
The energy intensity of water electrolysis—more than 50 megawatt-hours per tonne of hydrogen produced—calls for utility-scale renewable power: bulk wind (onshore or offshore), hydropower, or geothermal. Hydropower would seem a natural fit, as long as the water were not needed for human consumption.
Allam-Fetvedt cycle
A less energy-intense process now being commercialized is the Allam-Fetvedt cycle. Few outside the oil and gas industry have heard of it, not least because it was only recently invented, in 2010 by the British-American engineering team of Rodney Allam and Jeremy Fetvedt.
They set out to find a way to thermally extract hydrogen from a natural gas feed, which is mostly methane, without emitting CO2. They used a process called oxycombustion, in which the input gas is burned in the presence of a very hot, pressurized mixture of carbon dioxide and oxygen. In a commercial plant, the oxygen would be supplied from conventional air separation. This supercritical (or ‘transcritical’) CO2 acts as the working fluid to extract hydrogen. The exhaust gas, now a syngas, is passed through a heat exchanger which transfers its heat to pressurized CO2 flowing the other way to power the oxycombustion. In this way, the supercritical CO2 forms a closed loop. Hydrogen is extracted through a water gas-shift reaction. Excess CO2 from the methane combustion is conveniently already pressurized, allowing it to be used for other processes or sent directly for sequestration, thereby avoiding atmospheric emissions.[8]
The fossil-fuel industry jumped on this invention as a way to continue supplying natural gas for both clean electricity generation and emerging fuels—hydrogen and ammonia. A 50-MW test facility was built in Texas in 2018 to prove the technology, following which a company created to bring it to market, 8 Rivers, announced the development of a hydrogen and ammonia plant in the same state to produce 880,000 tonnes of hydrogen per year beginning in 2027. The synergy between the Allam-Fetvedt process and ammonia production is that they can share the same heat loop.
Whether a market exists for all the pressurized CO2 produced is an open question. Yet, even at this early commercial stage, the process is about 58% energy-efficient, requiring about 28 MWh of energy per tonne of hydrogen produced.[9]
Solid oxide water electrolysis
Another technology entering commercialization is solid oxide water electrolysis. This process splits water using a solid oxide electrolyte (typically zirconium dioxide, ZrO2) at high temperatures (600°C to 900°C). It is essentially a solid-oxide fuel cell running in reverse. The electrolyzer can use excess heat from the Haber-Bosch process to reduce electricity consumption. It also has the potential to simultaneously extract nitrogen from the air, thereby bypassing the air separation process.
Neat! The only snag is that it currently costs around 39 MWh per tonne of hydrogen produced, although that number should come down as economies of scale are achieved. Companies such as Bloom Energy, Fuel Cell Energy and Haldor Topsøe are now deploying commercial units.
This energy cost translates into market costs ranging from $3 to $8 per kilogram of hydrogen, according to the International Energy Agency, which quotes $1 to $2 per kg for hydrocarbon-produced hydrogen with carbon capture.[10]
Methane pyrolysis
Costing about the same energetically as the Allam cycle, methane pyrolysis is arguably a much simpler technology. It also produces solid carbon as a by-product rather than pressurized CO2. As the name implies, it uses intense heat (700°C to over 1100°C) to split hydrogen from methane. Mediated by a metal or carbon catalyst, CH4 is adsorbed onto the surface of the catalyst, where hydrogen atoms are progressively stripped off it, leaving solid carbon. Depending on the reaction conditions, this carbon can take the form of carbon black, graphite or even nanotubes, all of which have industrial uses.
The non-methane hydrocarbons present in a typical natural gas feed, such as ethane, propane and ethylene, seem to be converted to methane at these elevated temperatures. Hydrogen sulfide, also found in natural gas in small amounts, may help the catalytic conversion.
Various processes have been proposed for industrial scale-up, from conventional fluidized-bed production to a novel liquid bubble column reactor, but as yet the technology remains in development, with an average estimated energy cost of about 28 MWh per tonne of H2.[11]
Microbial electrolysis
Rather than relying on hot, pressurized industrial processes to extract hydrogen, why not harness the power of nature? This is the thinking behind microbial electrolysis, which employs microbes to convert organic chemicals into hydrogen. The same technology working the other way—a microbial fuel cell—can consume hydrogen to generate electricity. You can imagine, for example, a bio-powered community energy plant that would generate hydrogen for ammonia storage during periods of low demand, then convert the ammonia into hydrogen for use during periods of high demand.
The bio-power for such a facility can be wastewater or degraded biomass. Although a microbial electrolysis cell produces carbon dioxide as a by-product, it is not a net emitter of CO2 if the feedstock is organic in the first place.
Unlike their industrial-chemical brethren, microbial electrolysis cells operate at close to ambient temperatures and pressures, requiring only a modest electrical current. Yet even with efficiencies in the lab greater than 100 percent, it is estimated they would require around 130 MWh of energy to produce one tonne of hydrogen: very slow and expensive. The challenge, clearly, is how to scale them to industrial applications.[12]
Other interesting lab projects
Many other technologies trying to make it out of the lab face an equally uncertain future.
Some teams are trying to produce hydrogen photoelectrochemically. Seawater photocatalysis is the use of a solar furnace to split seawater into hydrogen and oxygen over a catalyst, such as titanium dioxide. The conversion efficiency is rather low: about 16 percent.[13]
Another avenue of investigation is to apply the principle of coal gasification to biomass. Biomass gasification is a thermochemical process in which a hot gas or gas-steam mixture is passed over biomass to release hydrogen and other products (CO, CO2, CH4 and N2), all of which can, in theory, be used in various ways to make ammonia. To-date, though, no operating plants employing this technique have been developed.[14]
Dispensing with Haber-Bosch
All this hydrogen-generating activity is very interesting and useful but the hydrogen itself still serves an industrial process—Haber-Bosch—that is a hundred years old. Surely modern technologies can do better?
The answer is yes, but none of them have yet been deployed, so we’re stuck with Haber-Bosch for at least a little while longer.
Electrochemical ammonia synthesis
Far and away the most promising non-Haber technology is electrochemical ammonia synthesis. It operates under mild conditions, unlike the energy-thirsty Haber-Bosch, and is flexible and scalable—two features essential to integration with renewable energy sources, whose output varies over time. For these reasons, it is an area of intensive research and development.
Electrochemical ammonia synthesis employs similar techniques to the production of hydrogen by electrolysis, taking a feed of nitrogen from an air separation unit and a feed of freshwater to supply hydrogen.[15] The by-product is oxygen, which can be emitted (carefully, because it is highly flammable) or pressurized for other uses.
2N2 + 6H2O + e– ⇌ 4NH3 + 3O2
Two techniques in particular are entering the demonstration phase.
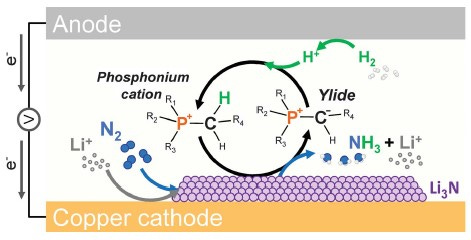
The electrochemical nitrogen reduction reaction (eNRR) adsorbs nitrogen onto an electrocatalyst, which enables two simultaneous processes: the breaking of the strong triple bond that holds a molecule of nitrogen together, and the progressive addition of protons and electrons to the unbound nitrogen atoms to produce ammonia.
In the indirect or mediated NRR, a redox mediator (typically lithium ions) is deposited on a copper substrate, where it reacts with nitrogen to form lithium nitride (LiN3). Hydrogen is added via an anion (such as a hydroxyl ion, OH–) to produce NH3, which is released along with the anion and the lithium mediator, the latter being returned to the substrate for the next cycle.
In theory, eNRR could use as little as about 5.5 MWh per tonne of ammonia produced, assuming a 100% efficient electrochemical process. The best electrochemical efficiency to come out of the lab so far is about 70 percent, which translates into an energy requirement around 8 MWh per tonne.[16]
Urea extraction and decomposition
A novel way of producing ammonia is from wastewater, which naturally contains large amounts of urea. As you can see from its chemical formula, which is CO(NH2)2, urea already contains both the nitrogen and hydrogen for ammonia. The only snag is that it also contains carbon and oxygen, which potentially means CO2 emissions.
In the lab, ammonia can be produced via decomposition into ammonium cyanate (NH4OCN) at temperatures above 160°C. The by-product is isocyanic acid (HNCO).
CO(NH2)2, ⇌ NH3 + HNCO
This isocyanic acid can produce additional ammonia in the presence of steam, releasing CO2.
HNCO + H2O ⇌ NH3 + CO2
Overall, the process yields two molecules of ammonia from each molecule of urea, emitting one molecule of carbon dioxide along the way. Although CO2 is released, as with microbial electrolysis and biomass gasification, it is not a net emission but simply a recirculation of carbon already in the natural cycle.
Because the process operates at moderate temperatures and uses an existing waste stream, its energy costs are small: about 0.62 kWh per kilogram of ammonia produced.[17] Given that ammonia’s lower heating value is 5.2 kWh per kg, this means that the process could yield nearly ten times as much energy from ammonia as is needed to make it. The result, while tantalizing, needs to be verified in scaled-up demonstration processes. Another uncertainty is whether or how wastewater treatment plants could be adapted to harvest ammonia at the volumes needed in a future ammonia energy economy. While promising, this technology might in the long run play a niche role.
Other ammonia production technologies emerging from the lab
A flurry of activity in labs around the world has produced all kinds of other ammonia-synthesis technologies.
Substances that contain both nitrogen and hydrogen are receiving special attention, such as nitric acid (HNO3) which can produce ammonia via electrolysis.[18] Electrochemical ammonia synthesis also can be induced or enhanced using electromagnetic fields.[19] Among biological processes, nitrogenase organisms and biomimetic catalysts can be used to create ammonia from nitrogen, the hydrogen being supplied from water electrolysis.[20]
These are all but a small sample of the innovations that could transform the energy economy in years to come.
Costs and perspective
How do the newcomers stack up against the established fossil-fueled technologies and against one another? Is there a case for modifying existing plants to reduce or eliminate their carbon footprints? Could they eventually be replaced with completely new facilities, producing at vastly larger scales?
The table below attempts to summarize the energy costs of the established and emerging technologies, based on data in the current literature. It’s very much a back-of-the-envelope calculation, bearing in mind also that emerging technologies carry higher costs than established ones, just by virtue of not having achieved the requisite economies of scale. With these caveats, however, it gives a rough idea of what is possible.
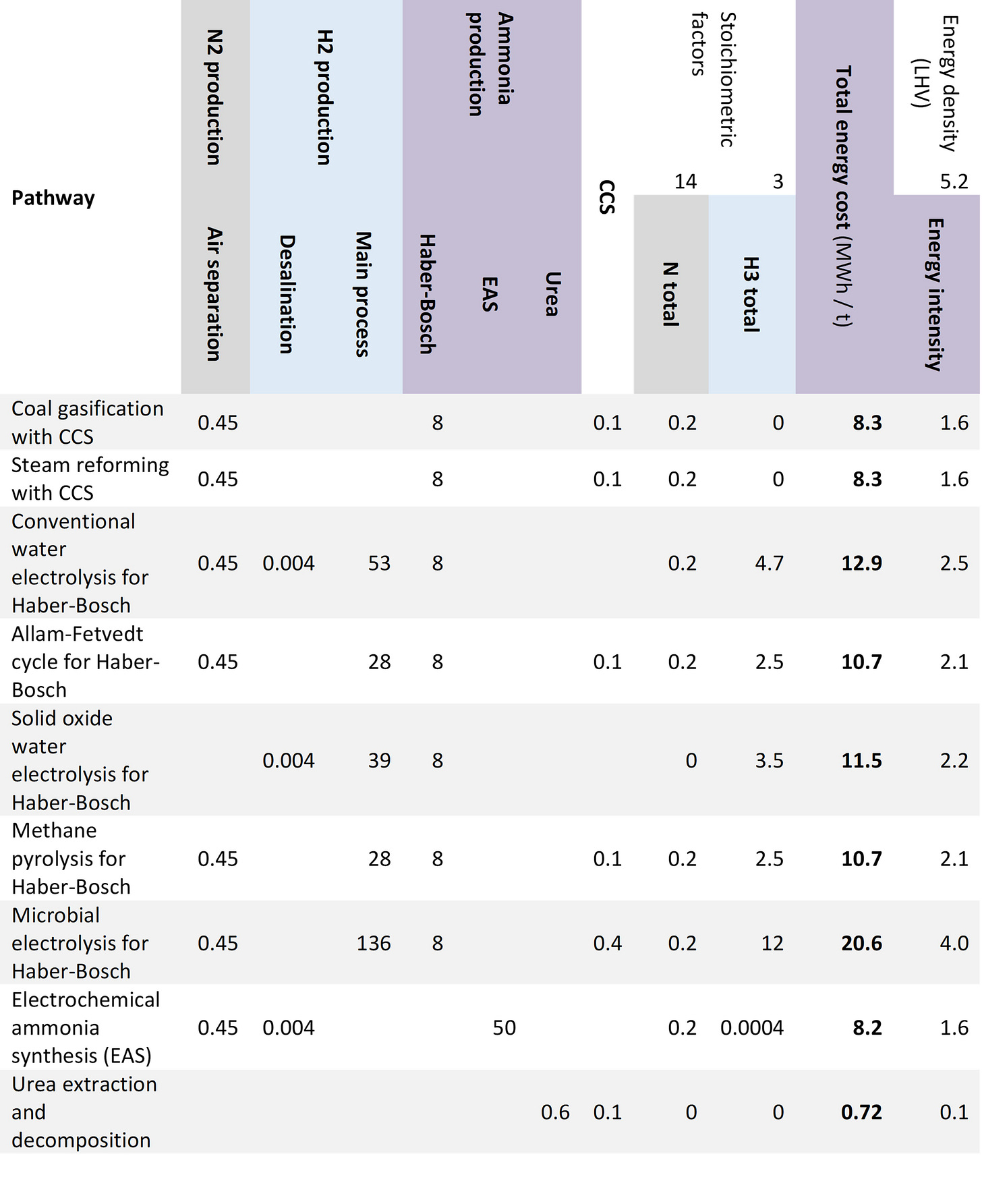
As the table shows, the total energy cost for conventional ammonia production via fossil fuels is lower than all the alternatives except for urea extraction—with the caveat that the latter is still a theoretical cost that would have to be validated in practice. Bear in mind that the fossil energy costs in the table do not include energy costs associated with the extraction and transport of the fuels themselves, so in that way they are an underestimate.
On an energy basis, electrochemical ammonia synthesis already looks cost-competitive with conventional synthesis. The other emerging technologies, while somewhat more costly, should become competitive with conventional production as they scale up, particularly if encouraged with government incentives.
Naturally, the role of incentives is to represent the benefit of low- or zero-carbon production in the market price of the commodity produced, thereby encouraging the replacement of dirty technologies with clean ones. With that in mind, governments should not only provide incentives for emerging clean technologies but also simultaneously reduce subsidies for fossil fuels. This would increase the cost of ammonia through conventional production, which would raise its price in the market. Although often considered politically unpopular, it would accelerate the transition. As I showed in my recent book, A Planetary Economy, if all the fossil-fuel subsidies in the US hypothetically were transferred onto renewables, the price of renewables actually would go negative.[21] That’s how much the fossil-fuel industry continues to be supported.
In money terms, conventionally produced ammonia is pretty inexpensive, roughly $0.06 per kWh[22] or about the same as a gallon of gasoline in the US.[23]
Even if carbon-free ammonia represents only a tiny fraction of all ammonia produced today, the indications are clear that ammonia production not only can be decarbonized but is already beginning to be decarbonized.
Processes such as the Allam-Fedvedt cycle and methane pyrolysis have the potential to not only bolt onto existing Haber-Bosch plants but also onto conventional gas-fired power generation. Any large plant having a natural gas feed can be adapted to produce ammonia via these processes, the ammonia then being fed into turbines to generate electricity.
Water electrolysis for hydrogen production, as well as direct electrochemical ammonia syntheses, are also technically and economically feasible, at a variety of scales, and these technologies have the added advantage of being locatable anywhere ammonia could be produced using renewable power.
In fact, the expansion of an ammonia-based energy economy broadens the very definition of 'renewable power’ because now the processes that make carbon-free ammonia can be powered by ammonia-based energy generation, whether thermal or electrolytic, as part 1 described. The creation of a new ammonia energy loop will render the existing fossil-fuel loop redundant.
So, on the face of it, the energy economy could become almost completely ‘ammonized’, although it might take two or three decades. How would it be different from today’s energy economy? Statistics and dry generalizations can make a compelling economic case, but they don’t show how the whole system is interconnected. For that, we need examples: stories that bring the vision to life. These are the subject of Part 3, next.
Works cited
Allam, R. et al. (2017) Demonstration of the Allam Cycle: an update on the development status of a high efficiency supercritical carbon dioxide power process employing full carbon capture. Energy Procedia, 114: 5948–5966.
Aneke, M. and Wang, M. (2015) Potential for improving the energy efficiency of cryogenic air separation unit (ASU) using binary heat recovery cycles. Applied Thermal Engineering, 81: 223–231.
Asiain-Mira, R. et al. (2022) Hydrogen production from urea in human urine using segregated systems. Water Research, 222: 118931, 1–10.
Egerer, J. et al. (2023) The economics of global green ammonia trade — “shipping Australian wind and sunshine to Germany”. Applied Energy, 334: 120662, 1–15.
Escapa, A., San Martín, M.I. and Morán, A. (2014) Potential use of microbial electrolysis cells in domestic wastewater treatment plants for energy recovery. Frontiers in Energy Research, 2 (19): 1–10.
European Commission (2007) Reference Document on Best Available Techniques for the Manufacture of Large Volume Inorganic Chemicals - Ammonia, Acids and Fertilisers. https://eippcb.jrc.ec.europa.eu/reference/large-volume-inorganic-chemicalsammonia-acids-and-fertilisers.
IEA (2021b) Global Hydrogen Review 2021. IEA Report, November 2021, International Energy Agency, Paris.
Li, Y. et al. (2022) Electrolyte-assisted polarization leading to enhanced charge separation and solar-to-hydrogen conversion efficiency of seawater splitting. Nature Catalysis (online). https://doi.org/10.1038/s41929-023-01069-1.
Liu, C. et al. (2017) Ambient nitrogen reduction cycle using a hybrid inorganic–biological system. Proceedings of the National Academy of Sciences, 114 (25): 6450–6455.
MacFarlane, D.R. et al. (2020) A roadmap to the ammonia economy. Joule, 4: 1186–1205.
Moon, Y.H. et al. (2022) Recent Advances in Electrochemical Nitrogen Reduction Reaction to Ammonia from the Catalyst to the System. Catalysts, 12: 1015, 1–19.
Murison Smith, F.D. (2020) A Planetary Economy. Palgrave Macmillan, New York.
Radhika, D. et al. (2022) Microbial electrolysis cell as a diverse technology: overview of prospective applications, advancements, and challenges. Energies, 15: 2611, 1–19.
Reale, F. (2023) The Allam Cycle: a review of numerical modeling approaches. Energies, 16: 7678, 1–22.
Rousseau, R. et al. (2020) Microbial electrolysis cell (MEC): strengths, weaknesses and research needs from electrochemical engineering standpoint. Applied Energy, 257: 113938, 1–18.
Sánchez-Bastardo, N., Schlögl, R. and Ruland, H. (2021) Methane pyrolysis for zero-emission hydrogen production: a potential bridge technology from fossil fuels to a renewable and sustainable hydrogen economy. Industrial and Engineering Chemistry Research, 60: 11855–11881.
Smith, C., Hill, A.K. and Torrente-Murciano, L. (2020) Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy & Environmental Science, 13: 331–344.
Suryanto, B.H.R. et al. (2021) Nitrogen reduction to ammonia at high efficiency and rates based on a phosphonium proton shuttle. Science, 372: 1187–1191.
Tornatore, C. et al. (2022) Ammonia as green fuel in internal combustion engines: state-of-the-art and future perspectives. Frontiers in Mechanical Engineering, 8: 944201, 1–16.
Zhao, Y. et al. (2019) An efficient direct ammonia fuel cell for affordable carbon-neutral transportation. Joule, 3: 2472–2484.
[1] MacFarlane et al. (2020) p. 1187.
[2] Aneke and Wang (2015); maziak.co.uk.
[3] Egerer et al. (2023) p. 4.
[4] MacFarlane et al. (2020), pp. 1188–1189 and European Commission (2007) referenced therein. The EC document gives a range of 27.6 to 31.8 GJ / tonne on p. 93, defined as a ‘net’ energy consumption, which is assumed to be net of the exothermic heat of the ammonia synthesis reaction itself.
[5] Egerer et al. (2023) p. 3.
[6] Egerer et al. (2023) p. 4.
[7] Energy requirements for desalination vary by technology but the most common, reverse osmosis, needs about 4 kWh / m3 (= 1 tonne) of freshwater produced. By comparison, a typical water electrolysis plant will use upwards of 50 MWh of electricity to produce 1 tonne of hydrogen.
[8] Allam et al. (2017) pp. 5950–5951.
[9] Reale (2023) p. 4.
[10] IEA (2021b) p. 7.
[11] Sanchez-Bastardo et al., (2021) pp. 11870–11872.
[12] Escapa et al. (2014), Liu et al. (2017), Rousseau et al. (2020), Radhika et al. (2022).
[13] Li et al. (2022); also Yusuf and Ibrahim (2017), cited in Tornatore et al. (2022).
[14] Tornatore et al. (2022) p. 4.
[15] Moon et al. (2022) p. 1.
[16] Suryanto et al. (2021). See also MacFarlane et al. (2020) pp. 1190–1191. Suryanto et al. achieve this electrochemical efficiency using a feed of pure hydrogen. Whether the same can be accomplished with a feed of water is an area of active inquiry (MacFarlane, pers. comm.). If not, an upstream water electrolysis step would be needed that would increase the overall energy cost to around 13 MWh / tonne.
[17] Asiain-Mira et al. (2022) pp. 6–7.
[18] Long et al. (2020) cited in Tornatore et al. (2022).
[19] Chehade and Dincer (2021) cited in Tornatore et al., (2022).
[20] MacFarlane et al., (2020).
[21] Murison Smith (2020) p. 404.
[22] MacFarlane et al. (2020) p. 1197.
[23] Zhao et al. (2019) p. 2477.